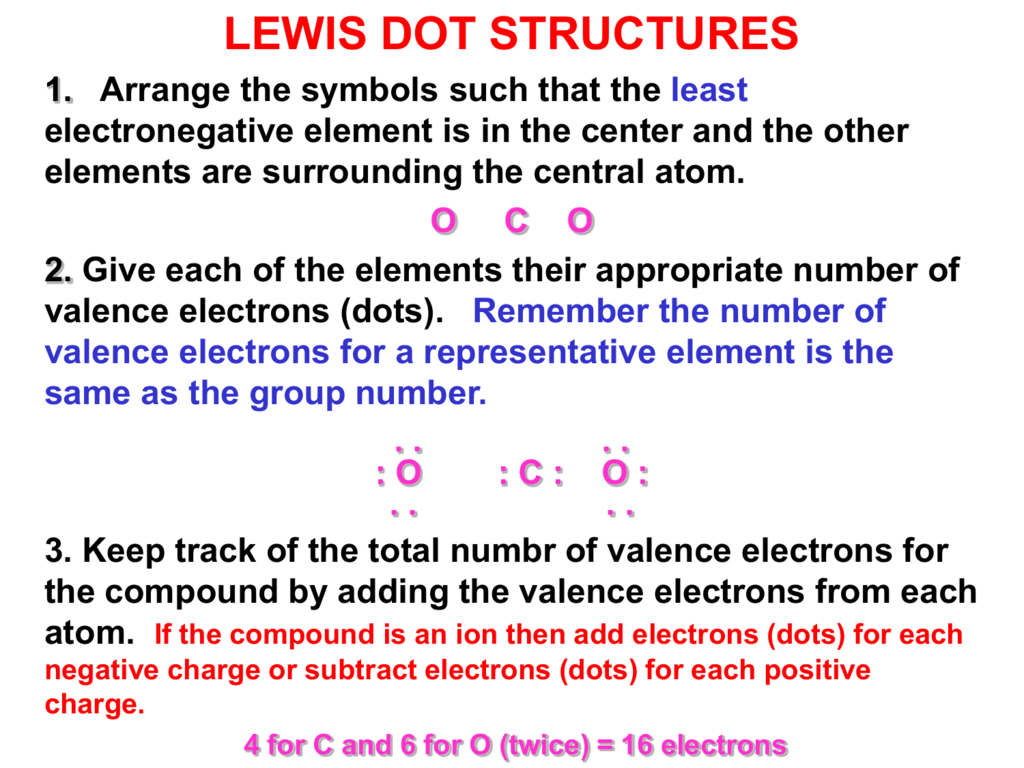
To find electronegativity either rely on periodic table trends or consult a table that lists electronegativity values. CO 2 Total 16.

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421
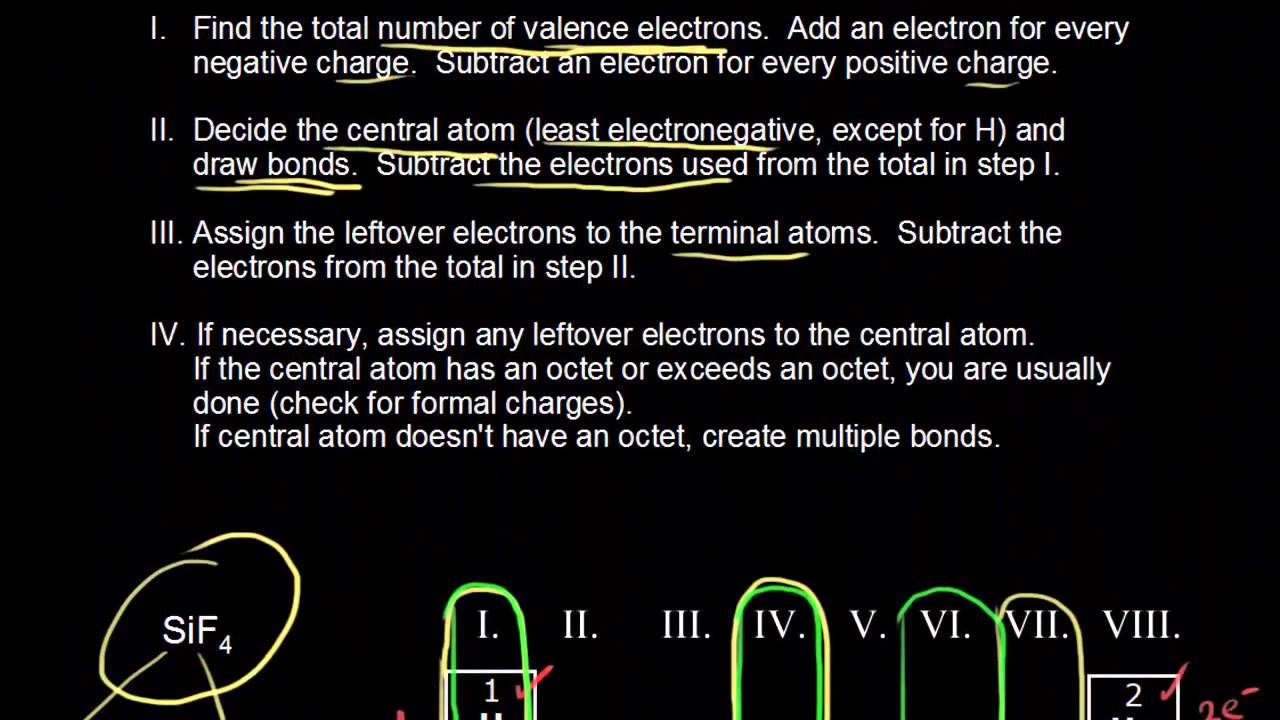
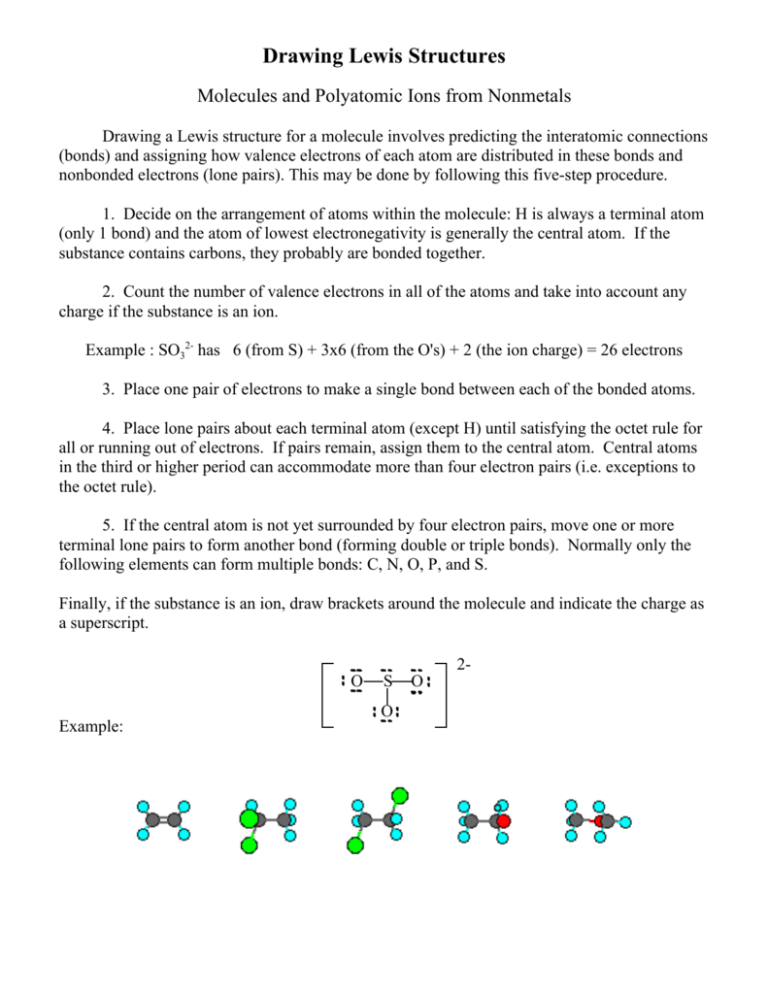
Here are the steps that I follow when drawing a Lewis structure.

. See answer 1 Best Answer. Determine the total number of electrons available for bonding. If you are drawing the Lewis structure of a cation or an anion it WILL.
Chemistry questions and answers. Atom with the fewest electrons b. When constructing a Lewis diagram keep in mind the octet rule which refers to the tendency of atoms to gain lose.
The core atom in the BCl3 Lewis structure is boron which is bonded to the three chlorine atoms by single bonds B-Cl. Decide which is the central atom in the structure. In drawing Lewis structures for relatively small molecules and polyatomic ions the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms.
In drawing the Lewis structure the central atom is generally the. Now as you know the concept of how the Lewis structure is constructed and what is its importance. C Si N P S O.
The least-electronegative atom is central except for hydrogen which is never central. So if you have covalent compound with C and P then C is the central atom because it comes first on the list. Usually the central atom will be the one that has the most unpaired valence electrons.
The central atom in a Lewis structure is usually the least electronegative atom. The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. What is the formal charge on the N.
The atom with the least electronegative charge should. Let us quickly hop on to the next segment which is understanding the Lewis structure of CCl4. With the help of three single bonds it already shares six electrons.
Regarding this how do you find the central atom when drawing a Lewis structure. Draw a trial structure by putting electron pairs around every atom until each gets an octet. In drawing a Lewis structure the central atom is the a.
How to Draw a Lewis Dot Structure Step 1. Ozone Valence electrons of oxygen 6. Calculate the total number of valence electrons in the molecule by adding the valence electrons from each atom.
This list will cover your needs on about 95 of questions you encounter for e lectron d ot s tructures Lewis Structures. Draw a skeleton structure in which the other atoms are single-bonded to the central atom. The central atom of a molecule is usually the least electronegative atom or the atom with the highest valence.
Lewis dot structures can be drawn to show the valence electrons that surround an atom itself. June 21 2021 by Admin. Furthermore What is the formal charge.
The central atom is also usually the least electronegative. 8 After you have completed your Lewis structure CHECK FOR OCTETS. If all atoms have an octet calculate formal charges for all atoms see below for method.
That will normally be the least electronegative atom C. Each step of drawing lewis structure of H 2 O are explained in this tutorial. What is the formal charge on CL in the following Lewis structure So the correct answer is 3.
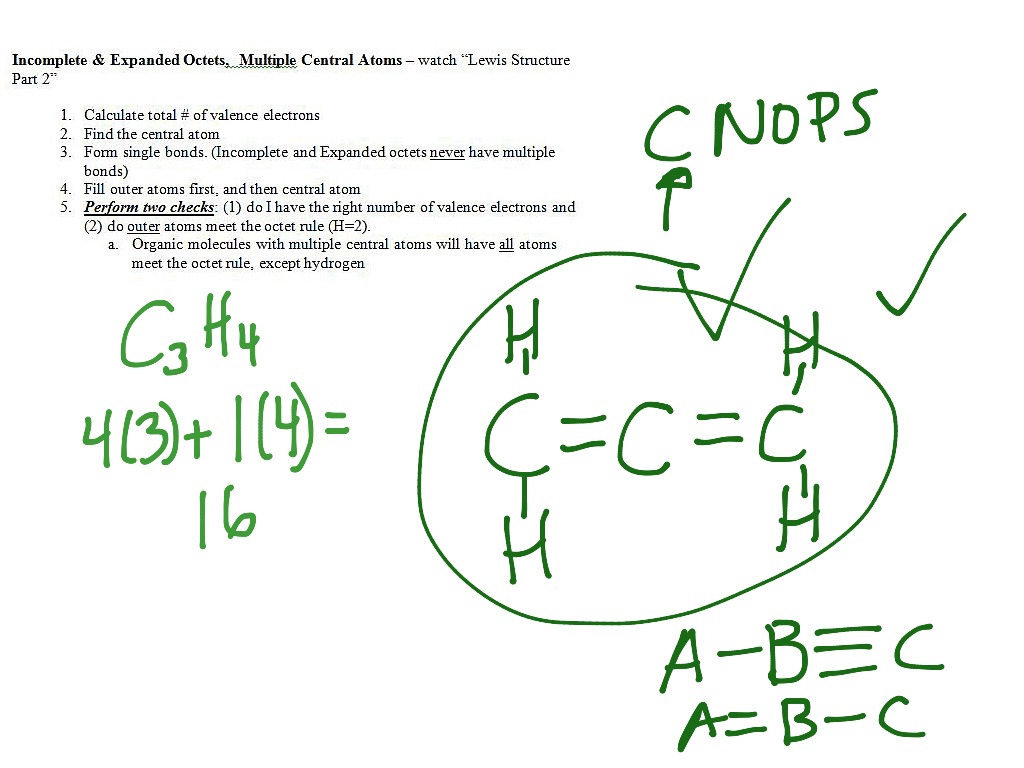
A-1 B 0 C 1 D 2 E-2 23 Draw the best Lewis structure for the free radical NO2. Determine how many electrons must be added to central element. Write the Lewis structure for CH2O where carbon is the central atom.
The central atom form 1 single bond 1 double bond with oxygen atoms. That list is. Lewis structure of water molecule contains two single bonds around oxygen atom.
Determine which atom will be the. If an atom occurs only once this atom is. Best Answer Copy The central atom in a Lewis structure is usually the least electronegative atom.
These elements are also in priority order. Complete the octet of ench atom. What is the formal charge on the central Cl atom.
H 2 O lewis structure. Oxygen Lewis dot structure Atom The Lewis structure of the oxygen atom is relatively easier to show as it does not involve any sharing or transference of electrons. Rules for Drawing Lewis Structure.
As oxygen atom Atomic number 8 and electronic configuration 26 belong to group 16 in the periodic table it will be surrounded by. In the lewis structure of H 2 O there are two single bonds around oxygen atom. 22 Draw the best Lewis structure for Cl3.
When drawing lewis structures which atom is usually the central atom. The Lewis structure is shown in the image below. It is also the atom having low electronegativity.
Place least electronegative element in center and draw single bonds from the central atom to other atoms. The diagram of the oxygen atom shows the valence electron for the element. Now as we continue to review how to draw Lewis structures we must decide which atom will be drawn in the center of our Lewis structure.
I usually prefer to teach my students this way at first to just get them rolling. Draw correct Lewis structures that obey the octet rule for each of the following. In every compound there is a central atom in the case of CCl4 the central.
The unpaired electron pair on central atom 1. Atom with the greatest mass c. Atom with the highest atomic number d.
Draw basic Lewis structure for each atom. For HCN and Hyco. If all of the atoms usually form the same number of bonds the least electronegative atom is usually the central atom.
Carbon is the central atom Assign tone pan radical elections and some changes where appropriate 0 НС Marvin 35 OHelp H-CEN Edit drawing b H2CO Marvin JS Help O O Edit. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. A Lewis diagram is a representation of the valence shell electrons in a molecule where each atom participating in the bond tries to acquire the octet structure.
Them place remaining electrons on the central atom IF the central atom is a Period 3 or greater element. -Hydrogen cannot be central. Except for HCN and Hyco the first atom listed is the centralatom.
From the Lewis structure it can be concluded that-The unpaired. Let us not waste time in understanding the structure of CCl4. We have found the total number of valence electrons.
Eight electrons come from one double bond pair of B-Cl and two single bond pairs of B-Cl on the boron central atom of BCl3. Only the valence electrons are shown by dots in the Lewis structure. The formal charge on central Cl atom is -1.
Determine the total number of valence electrons to be depicted in the Lewis diagram.
Drawing Dot Structures Video Khan Academy

Step 1 Identify The Central Atom And Draw It S Lewis Structure This Is The Element With The Lowest Number Of Atoms In The Formula Draw A Lewis Dot Diagram Ppt Download

How To Draw Lewis Structures For A Molecule With One Central Atom No Octet Rule Exceptions Chemistry Study Com
Rules For Drawing Lewis Structures
Lewis Structures Multiple Central Atoms Science Chemistry Chemical Bonds Showme
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
0 komentar
Posting Komentar